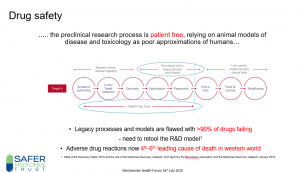
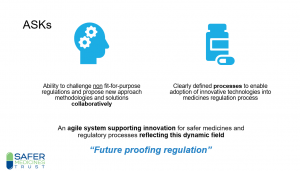
“The future for UK regulation of medicines, medical devices and clinical trials – transparency and public trust, safety and enforcement, and support for innovation” was the subject of a recent Westminster Health Forum meeting at which our Director, Dr Jan Turner described why innovation and the development of methods to ensure safe and effective medicines, is imperative for the UK now and importantly, post Brexit. A recording of the talk is here and Dr Turner’s slides are here