Solidarity PLUS: an international clinical trial to improve Covid-19 medicine discovery
Although vaccination has reduced markedly the UK population risk of serious illness and fatality during the ongoing third wave of the Covid-19 pandemic, it has become clear that vaccination does not provide complete protection against serious illness or prevent virus transmission. Additional medicines, which can block Covid-19 viral infection and transmission and can treat the many adverse symptoms that arise in susceptible individuals, continue to be needed urgently. Many potentially useful drugs have been proposed. However so far relatively few have been shown to be effective.
A key bottleneck has been the need to undertake rigorous trials, which are essential to differentiate between effective and ineffective treatments. Conventional clinical trials require many months, or years, to complete and typically cost up to tens of millions of pounds per medicine. Alternative approaches are needed, which are quicker and cheaper and yet equally rigorous. The opportunity to undertake quicker and cheaper clinical studies was demonstrated in 2020 by the UK’s RECOVERY trial, which demonstrated that the anti-inflammatory drug dexamethasone greatly reduced fatalities in Covid-hospitalised patients.
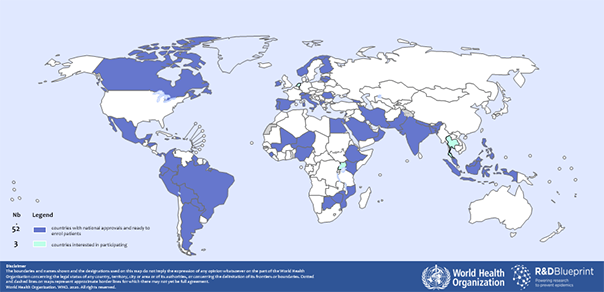
The World Health Organisation has now announced a major international trial which will enable researchers in 600 hospitals, located in 52 countries, to assess multiple treatments at the same time and using a single protocol. This impressive international collaboration (Solidarity PLUS) will utilise a single adaptive protocol and will standardise the evaluation methods using in the different hospitals. It will enable useful drugs to be identified quickly and effectively and will ensure that ineffective drugs can be replaced promptly with alternative candidates throughout the course of the trial. WHO’s approach is expected to improve greatly the speed with which better treatments for Covid-19 infection, and Covid-induced illnesses, can be identified and implemented.

