New Approach Methodologies in COVID-19: systematic review into the neurological effects of SARS-CoV-2 infection presented at SOT (Society of Toxicology) Congress 2022
By Rebecca Ram
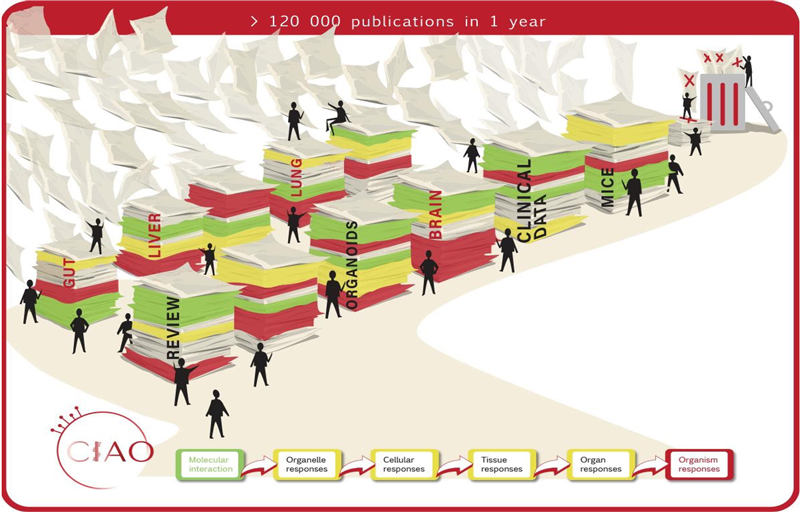
Launched nearly two years ago, the CIAO project (Modelling the Pathogenesis of COVID-19 using the Adverse Outcome Pathway Framework) involves the collaboration of scientific experts to identify the chemical and biological events involved in the body’s response to the SARS-CoV-2 virus. Expert working groups are established across a number of areas, for example acute respiratory distress, lung fibrosis, neurological, liver and cardiovascular injury.
At the US Society of Toxicology (SOT) 2022 congress in San Diego last week, research was presented by the project’s literature review working group on the key central nervous system (CNS) events in COVID-19. Using a systematic review (SR) approach, the group, led by the EBTC (Evidence Based Toxicology Collaboration) identified and analysed data relevant to the neurological adverse outcomes of SARS-CoV-2 infection and to further identify potential gaps in knowledge to inform the COVID-19 AOP framework. The results were presented by Donna Macmillan in a poster at the SOT congress, including quantitative analysis and data extraction demonstrated in a systematic evidence map. The SR protocol used has been published as a general approach and guidance to other research areas.
The Adverse Outcome Pathway (AOP) is a game changing pathway-based concept, developed over the last two decades to provide highly mechanistic information on the effects of a ‘stressor’ (for example any chemical or virus – in this case the SARS-CoV-2 spike protein) triggering a ‘molecular initiating event’ (MIE) and progressing through higher level ‘key events’ (KEs) at cell, organ and tissue level; the relationships between those key events (KERs) through to adverse outcomes observed in the individual and potentially, populations.
The AOP was originally intended to establish toxicity pathways in response to chemicals, but as all diseases, bacteria and viruses act in a similar way to trigger MIEs, AOPs have application across chemical safety, basic and applied/translational disease research. Key events and adverse outcomes are not necessarily unique to one AOP and so can be connected to form almost infinite adverse outcome ‘networks’.
Data resulting from different types of test method can be integrated to map events in AOPs, for example in silico (computational) models for chemical screening (from simpler analysis models through to artificial intelligence, or AI, derived data from machine learning algorithms), in vitro assays (advanced human cell based models such as stem cells or 3D organoids) to assess biological activity, as well as a variety of human based data (e.g. clinical trials, biomonitoring and ‘real world data’ from epidemiological and observational studies). Combinations of these methods are termed ‘New Approach Methodologies’ (NAMs) and they are designed to overcome limitations in the predictive capabilities of conventionally used methods (‘in vivo’ animal models). Case study evidence in NAMs continues to emerge, aiming to provide practical guidance and overcome the bottleneck in achieving regulatory acceptance.
Although the basic AOP is visualised as a linear ‘left to right’ concept, the AOP can be used in any direction or started at any point and all AOPs are ‘dynamic’, as new and updated information can be added to them continuously. In simple terms, AOPs might be very broadly compared to jigsaw puzzles. As pieces (or ‘data gaps’) are filled, they show what is still missing.
Read our previous post on the CIAO project here
The poster above can also be accessed on the CIAO publications page
To find out much more on the successful outcomes of the project to date and how to become involved, visit https://www.ciao-covid.net/
Further information on AOPs, including useful training and educational resources can be found here

